当前位置
Sustainability of public cord blood banks and challenges of biotherapy applications
Background Trends
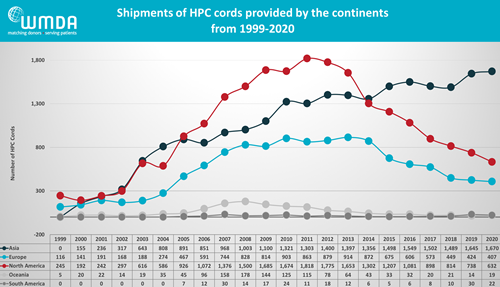
In recent years, the popularity of cord blood as a graft source for stem cell transplants has been waning in Western countries. Over the decade from 2011 to 2020, the World Marrow Donor Association (WMDA) reports that the annual number of cord blood transplants decreased by one third from 4110 to 27501. A graph of geographic trends in cord blood transplantation reveals a nearly 3-fold drop in North America, while at the same time cord blood utilization continues to rise in Asia. These trends have led to a serious discussion about the financial “sustainability” of cord blood banks in the western hemisphere. A potential new avenue of income for these banks is the biotherapies market, where public banks could provide raw materials and/or expert services for the manufacture of novel therapies using cells sourced from cord blood.
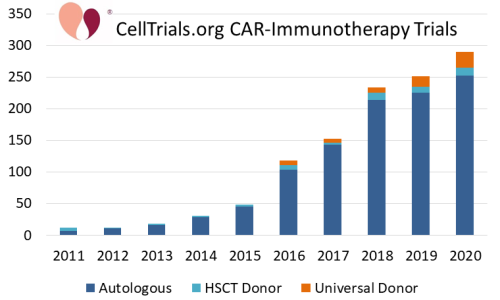
During the same time frame, immunotherapy treatments for cancer have been growing dramatically. One example is CAR-immunotherapy in which immune cells are genetically modified with Chimeric Antigen Receptors (CAR). The accompanying graph from CellTrials.org displays the number of CAR immunotherapy clinical trials (not just CAR-T) per year over the decade from 2011 to 2020. The number of trials per year increased 25-fold over this decade. Furthermore, the graph of clinical trials is color-coded by whether the cell donor for the CAR-immunotherapy is autologous, allogeneic matched, or allogeneic off the shelf. In trials where the donor was not specified, it was defaulted to autologous for T-cells and allogeneic for NK cells. Obviously, the vast majority of CAR-immunotherapy trials use autologous cells, and all of the FDA approvals to date have been for autologous CAR-T therapies3-7. Nonetheless, by the end of 2020, 13% of CAR-immunotherapy clinical trials used allogeneic cell sources, either from a matched donor or a universal donor product. Among the companies working in CAR-immunotherapy, we found that 35% were developing allogeneic products by late 20208.
Cord Blood Cells for Immunotherapy: Achievements
The rapidly growing market for cell-based immunotherapy has led some to question whether cord blood can be used as a source material for immunotherapy? The very short answer is YES. About a year ago, in October 2020, Parent’s Guide Cord Blood published a news article on the current uses of cord blood for immunotherapy. At that time, 123 recruiting clinical trials were using cord blood for advanced cell therapy. Among those recruiting trials, 17% were using cord blood to derive specialized immune cells, such as Natural Killer (NK) cells, regulatory T-cells (T-regs), or CAR-T cells9.
Several companies are developing immunotherapy product pipelines that are based on cells derived from perinatal sources. We showcase a few of these companies that have reached clinical trials in the accompanying table. This is meant to be an illustration, and is not a comprehensive list.
Institution | Cell Type | Cell Source | Sample Trial |
NK | Cord Blood | ||
NK | Cord Blood | ||
T-regs | Cord Blood | ||
NK | Placental Blood | ||
CAR-T | Placental Blood | ||
NK | Cord Blood | ||
NK | Cord Blood | ||
Henan Hualong | CAR-T | Cord Blood | |
MD Anderson (licensed Takeda) | NK | Cord Blood | |
Sidemu Biotech | NK | Cord Blood |
Cord Blood Cells for Immunotherapy: Challenges
We have already seen that cord blood can be used as the source material for immunotherapies. However, in order to contribute to the sustainability of public cord blood banks, this avenue of therapy would have to routinely require the release of cryopreserved donations. This leads us to discuss some of the strengths and weaknesses of the existing cord blood inventory in public banks, as a potential source for immunotherapy products.
It is very important to disentangle two related topics: One is whether fresh cord blood can be a starting material for biotherapies, whereas the other is whether the cryopreserved cord blood units in public banks can be a starting material for biotherapies. It is well established that cell counts decrease somewhat after cycles of freezing and thawing10,11, so what advantages could cryo-preserved cord blood units have over fresh collections?
The primary advantage of archived cord blood units is that they have already passed quality testing. For example, full results from infectious disease testing and sterility cultures usually take two weeks. Fresh cord blood cannot be held while waiting for test results, it would have to be processed, and any manufactured cellular products would have to be kept isolated from other biologic samples while waiting for culture results. By comparison, cord blood units preserved in public banks are fully vetted for human use. They are ready “off the shelf” (actually out of the freezer).
Moreover, when the starting material is cord blood units from a public bank that has FDA BLA approval, this streamlines the process for the immunotherapy company to obtain FDA approval of the final manufactured product12.
Those cord blood units in public banks which are big enough to transplant an adult have an average cost of $36,20013, making them an expensive raw material. But so far, the immunotherapy products that have come to market are at least a factor of ten more expensive14, so that purchasing cord blood units from a public bank is an acceptable cost of doing business. This may change as more immunotherapies are approved by the FDA, and the effort to lower their cost becomes a competitive factor.
On the flip side, the cost of acquiring cord blood units can be lowered if the immunotherapy company makes bulk purchases of cord blood units, or if the company purchases smaller cord blood units that are not suitable for transplant.
Another advantage that public cord blood banks possess is that they are designed to hold immune cells that cover a diverse inventory of HLA types15. This can be an advantage for any immunotherapy that is allogeneic but matched. However, very few developers have exploited this capability so far. Most of the immunotherapy products that are sourced from cord blood and have reached clinical trials are universal donor NK cells.
There are two challenges encountered when researching T-cell therapies sourced from cord blood. One is that the T-cell population of newborn cord blood is fundamentally different from the T-cells in adult blood, and the other is the manufacturing challenge of isolating T-cells from cryopreserved cord blood.
During pregnancy, the mother’s body tolerates the presence of a foreign invader, namely the baby, for nine months16. An enormous amount of research has gone into understanding the immunology of this tolerance. Part of the tolerance is mediated by the placenta, and part of it is enabled by the immature state of the fetal immune system. At birth, the newborn blood in the umbilical cord represents the fetal immune system. The T-cells in cord blood are mostly “naieve” and have not been “educated” in the thymus gland to recognize foreign invaders by expressing T-cell receptors (TCR)17,18.
There are potential advantages to using newborn cord blood for the development of T-cell therapies.
- Cord blood is very rich in regulatory T-cells, compared to adult blood, which is an advantage for any immunotherapy based on T-regs17.
- Cord blood transplants have demonstrated that cord blood has strong anti-leukemia activity for some patients19,20.
- We also know from cord blood transplants that cord blood immune cells trigger much less graft versus host disease (GvHD) than adult graft sources21. In theory this could help with the development of universal donor CAR-T products. At present, companies such as Allogene are trying to prevent GvHD in universal donor CAR-T by using gene editing to eliminate TCR in the T-cells of adult donors.
A manufacturing challenge for the development of T-cell therapies is the need to obtain a very pure sample of immune cells as starting material. CAR-T manufacturing usually starts with donor leukapheresis, with the goal of collecting about 1 billion white blood cells that are at least 99.5% pure22. There should be virtually no red blood cells (RBC) in the starting sample for CAR-T manufacturing, because RBC inhibit T-cell activation and proliferation23,24.
While it has become standard practice for both public and private cord blood banks to remove RBC before cryopreservation, most of the processing methods in standard use leave a residual RBC content of 10-20%25-29. One exception is PrepaCyte-CB processing, which has a residual RBC level near 1%26,28. Similarly, the CE-marked device TotiCyte has demonstrated greater than 99% removal of RBC29.
Hence, using cryopreserved cord blood as the starting point of a T-cell therapy would require putting the units through a washing and cell separation process post-thaw to remove the remnants of RBC. This labor step is not unique to cord blood, because any cryopreserved cell product must be washed post-thaw to remove cryoprotectant. However, the removal of RBC requires special effort because they rupture during freezing, a process called “lysis”, which makes it necessary to remove free hemoglobin and other cellular debris that can be toxic to patients, and meanwhile valuable cell types may be lost during the cleaning process30.
This article is not meant to be a comprehensive review, but to provoke discussion by enumerating the various challenges encountered when cryopreserved cord blood is considered as a source for biotherapies. This month, the Cord Blood Association is holding their annual meeting, and an entire session on September 23 is devoted to Cord Blood Banking Sustainability.
References
- World Marrow Donor Association 2020 WMDA Finance & Activities Report
- Verter F, Bersenev A. Growth of Allogeneic CAR-Immunotherapy. CellTrials.org Blog Published 2020-03-17
- FDA. FDA approval brings first gene therapy to the United States. FDA News Release Published 2017-08-30 [Kymriah]
- FDA. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. FDA News Release Published 2017-10-18 [Yescarta]
- FDA. FDA Approves First Cell-Based Gene Therapy For Adult Patients with Relapsed or Refractory MCL. FDA News Release Published 2020-07-24 [Tecartus]
- FDA. FDA Approves New Treatment For Adults With Relapsed Or Refractory Large-B-Cell Lymphoma. FDA News Release Published 2021-02-05 [Breyanzi]
- FDA. FDA Approves First Cell-Based Gene Therapy for Adult Patients with Multiple Myeloma. FDA News Release Published 2021-03-27 [Abecma]
- Bersenev A, Verter F. Where to Find Companies Developing Allogeneic CAR-Immunotherapy. CellTrials.org Blog Published 2020-10-24
- Verter F. Current uses of Cord Blood for Immunotherapy. Parent's Guide to Cord Blood Foundation Newsletter. Published October 2020.
- Querol S, Gomez SG, Pagliuca A, Torrabadella M, Madrigal JA. Quality rather than quantity: the cord blood bank dilemma. Bone Marrow Transplantation 2010; 45:970–978.
- Akel S, Regan D, Wall D, Petz L, McCullough J. Current thawing and infusion practice of cryopreserved cord blood: the impact on graft quality, recipient safety, and transplantation outcomes. Transfusion 2014; 54(11):2997-3009.
- Rao M. Cord Blood and the FDA. Parent's Guide to Cord Blood Foundation Newsletter Published January 2015.
- Verter F. RAND Corporation report on Public Cord Blood Banking Industry. Parent's Guide to Cord Blood Foundation Newsletter. Published November 2017
- Locke F, Lin JK. Are CAR T-Cell Therapies Worth the Costs? ASH Clinical News Published 2020-02-01
- Verter F. Be The Match® has a matching stem cell donor for most US patients. Parent's Guide to Cord Blood Foundation Newsletter. Published October 2014
- Loke YW. Life’s Vital Link: The Astonishing Role of the Placenta Oxford University Press 2013; ISBN:978-0199694518
- Rackaityte E, Halkias J. Mechanisms of Fetal T Cell Tolerance and Immune Regulation. Frontiers Immunology 2020; 11:588.
- Sedwick C. The Education of Mr. T. PLOS 2006; 4(4):e117
- van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci 2012; 109:2509–2514.
- Milano F, Nelson JL, and Delaney C. Fetal maternal immunity and antileukemia activity in cord-blood transplant recipients. Bone Marrow Transplantation 2013; 48:321–322.
- Rocha V, Wagner Jr. JE, Sobocinski KA, Klein JP, Zhang MJ, Horowitz MH, Gluckman E. Graft-Versus-Host Disease in Children Who Have Received a Cord-Blood or Bone Marrow Transplant from an HLA-Identical Sibling. NEJM 2000; 342:1846-1854.
- Allen ES, Stroncek DF, Ren J, et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion 2017; 57(5):1133-1141.
- Long K, Meier C, Bernard A, Williams D, Davenport D, Woodward J. T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion 2014; 54(5):1340-1347.
- Gerner MC, Bileck A, Janker L, Gerner C, Schmetterer KG. Packed red blood cells inhibit T-cell activation via ROS-dependent signaling pathways. J Biological Chemistry 2021; 296:100487
- Lapierre V, Pellegrini N, Bardey L, Malugani C, Saas P, Garnache F, Racadot E, Maddens S, Schillinger F. Cord blood volume reduction using an automated system (Sepax) vs. a semi-automated system (Optipress II) and a manual method (hydroxyethyl starch sedimentation) for routine cord blood banking: a comparative study. Cytotherapy 2007; 9(2):165-169.
- Basford C, Forraz N, Habibollah S, Hanger K, McGuckin C. The Cord Blood Separation League Table: a Comparison of the Major Clinical Grade Harvesting Techniques for Cord Blood Stem Cells. Int. J. Stem Cells 2010; 3(1):32-45.
- Solves P, Planelles D, Mirabet V, Blanquer A, Carbonell-Uberos F. Qualitative and quantitative cell recovery in umbilical cord blood processed by two automated devices in routine cord blood banking: a comparative study. Blood Transfusion 2013; 11(3):405-411.
- Cryo-Cell. PrepaCyte-CB Accessed 2021-09-09
- Drew J, Slaughter R, Klimentov A, Channon WA, Rees C, Harrington W, Watts M, Martin LA. TotiCyte, a Paradigm Shift in Stem Cell Isolation and Storage from Umbilical Cord Blood. Stem Cell Res Dev Ther 2021; 7:073
- Scaradavou A. Why red blood cells should be removed before cord blood storage. Parent's Guide to Cord Blood Foundation Newsletter. Published November 2014


