Jste zde
Cells4Life Brings Placenta Banking to the UK
Cells4Life has been authorised by the UK regulator, the Human Tissue Authority (HTA), to offer this service, giving parents access to a further two sources of perinatal stem cells at the birth of their new baby. These are the amniotic membrane and the chorionic villi, which can now be banked in addition to cord blood and cord tissue.
Cells4Life is the first private stem cell bank to store both of these tissue types selectively, rather than providing a generic placenta banking service. By banking all four, cord blood, cord tissue, amniotic membrane and chorionic villi (placental cells), Cells4Life believes parents gain the potential to access the widest range of treatments possible, as various regenerative therapies begin to transition from clinical trials to the clinic.
Speaking about the benefits of placenta banking, Lesley-Ann Martin, Chief Scientific Officer at Cells4Life, said “Research is moving rapidly, and placental banking opens up further potential therapeutic possibilities for parents looking to protect their child’s long-term health. Studies suggest stem cells from different tissue sources such as cord blood, umbilical cord tissue and of course placenta will have different potential therapeutic applications. In general, I would say that storing placenta alongside cord blood and tissue maximises the future access to these developing treatments and technologies.”
Lesley-Ann also adds: “The UK is one of the leading countries in stem cell research and development. We want to remain at the clinical forefront and our strong in-house R&D department will continue to spearhead the launch of processes that will help parents take advantage of new, exciting therapies that will begin to become available.”
Cells4Life has been an innovator in the perinatal stem cell field since its establishment in 2002. It is best known for the development of TotiCyte, a method for processing cord blood that gives an uplift in the number of stem cells retained at the point of therapy. Cells4life also offers a postnatal genetic testing kit called BabyInsight that lets you check your baby’s predisposition to certain intolerances and sensitivities so you can put measures in place at an early age.
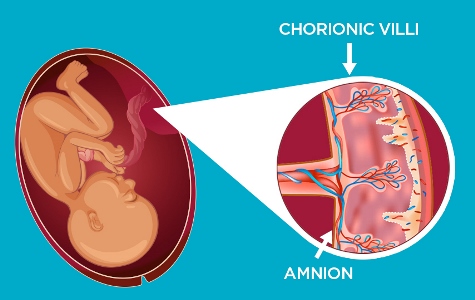
During pregnancy, the placenta nourishes your baby with everything it needs to grow while also removing waste prodcuts. However, its usefulness extends beyond birth. The placenta has two key components which are rich in different regenerative cell types: the chorionic villi and the amniotic membrane. Both of these tissues have powerful regenerative properties.
The chorion is the outermost layer of the placenta that comes in contact with mum’s womb. The chorion contains villi, which are small, tree-like structures that are involved in the exchange of nutrients from mum to baby and removal of waste during pregnancy. The chorionic villi are a rich source of mesenchymal stromal cells (MSCs) and these cells are being used to push the boundaries of conventional medicine. Clinical trials for numerous conditions such as arthritis, brain injury, cardiovascular conditions, diabetes and more are currently underway.
The amniotic membrane is the innermost skin of the placenta and forms a sac around the baby holding amniotic fluid. The thin layer of cells in the amnion have been used for over a century in a range of treatments for burns, ulcers, and wounds. Today, they are the subject of revolutionary clinical trials investigating therapies for premature babies, lung fibrosis and more.
The emerging consensus amongst scientists is that MSCs from different sources are likely to vary in the types of therapies for which they are best suited: “MSC origins may have significant impact on the therapeutic potentials of such cells and should be taken into consideration in clinical applications”1. This means that for some therapies, MSCs originating from the chorion may be preferred, while in others, amnion, or cord tissue MSCs might be used instead.
Another consideration when banking stem cells is to have more options for accessing new therapies. When an experimental therapy goes through clinical trials and is approved or patented by regulatory authorities, the approval requires the manufacturer of the product to consistently use the same cell source and same procedures that were in the request for approval. Parents that are seeking an alternative cell therapy for the same condition could turn to a different tissue source.
To find out more about placenta banking, visit Cells4Life.com.
References:
- 1. Zhu et al. Placental mesenchymal stem cells of fetal and maternal origins demonstrate different therapeutic potentials. Stem Cell Research & Therapy 2014; 5:48
- 2. Search of ClinicalTrials.gov, for keywords “Amniotic Membrane”, U.S. National Library of Medicine, 2022;
- 3. Search of ClinicalTrials.gov, for keywords “Chorionic Villi”, U.S. National Library of Medicine, 2022;


 What is placenta banking?
What is placenta banking?