Jste zde
Research on Allogeneic Cord Blood for Stroke
 The possibility of using cord blood stem cells to treat adult stroke patients is an area of research that holds enormous potential. Stroke affects one in every six people worldwide, and is the world’s second most common cause of death1-3. Any new therapy that helps stroke patients could have blockbuster sales. For the cord blood industry, a new application of allogeneic cord blood for a common indication could save the public banks that are financially struggling4,5.
The possibility of using cord blood stem cells to treat adult stroke patients is an area of research that holds enormous potential. Stroke affects one in every six people worldwide, and is the world’s second most common cause of death1-3. Any new therapy that helps stroke patients could have blockbuster sales. For the cord blood industry, a new application of allogeneic cord blood for a common indication could save the public banks that are financially struggling4,5.
The definition of a stroke is the sudden death of brain cells due to lack of oxygen, which happens when blood flow is disrupted in a region of the brain. In about 87% of strokes, the blood flow is disrupted by a blockage, and this is called an ischemic stroke, but stroke can also be caused by bleeding in the brain, and this is called hemorrhagic stroke1.
Stem cell therapies emerged as a paradigm for stroke about a decade ago6. Contrary to long-held beliefs, we now know that the brain can recover to some extent after injury, and stem cell therapy offers a potential for multimodal repair mechanisms. “Immediately after stroke, several events, including edema, deafferentation, and inflammation, occur around the infarct, and some early functional recovery can be attributed to the resolution of edema and inflammation. However, this is usually limited, and other processes, including immunomodulation, angiogenesis, endogenous neurogenesis, and altered gene expression, may be involved in the longer-term recovery of function”7.
According to the resource CellTrials.org, over the period from January 2011 through July 2019, 77 clinical trials have been registered worldwide to treat stroke with cell therapy, with 68% of the trials listed on ClinicalTrials.gov and 32% listed in 9 other trial registries8.
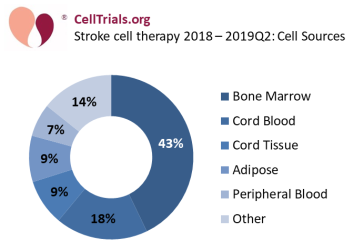 The most common source for cells used in stroke cell therapy is bone marrow, at 43% of these 77 trials (see Figure 1: Stroke cell therapy - cell sources). Some stroke therapies developed from bone marrow have a strong pipeline of development and are close to approval in their respective countries. For example, the Cleveland-based company Athersys has received Regenerative Medicine Advanced Therapy (RMAT) Designation from the FDA for their product MultiStem®. Athersys has begun a phase 3 trial NCT03545607 in June 2018 that plans to enroll 300 patients at 6 medical centers in the United States. Meanwhile, the Japanese company SanBio has completed a phase 2 trial NCT02448641 of their product SB623 Implant that was registered in 2015 and ran at 65 locations across the United States. That trial met its primary endpoints, and in April 2019 SanBio received a Sakigake Designation for SB623, which is a Japanese version of a fast track to approval.
The most common source for cells used in stroke cell therapy is bone marrow, at 43% of these 77 trials (see Figure 1: Stroke cell therapy - cell sources). Some stroke therapies developed from bone marrow have a strong pipeline of development and are close to approval in their respective countries. For example, the Cleveland-based company Athersys has received Regenerative Medicine Advanced Therapy (RMAT) Designation from the FDA for their product MultiStem®. Athersys has begun a phase 3 trial NCT03545607 in June 2018 that plans to enroll 300 patients at 6 medical centers in the United States. Meanwhile, the Japanese company SanBio has completed a phase 2 trial NCT02448641 of their product SB623 Implant that was registered in 2015 and ran at 65 locations across the United States. That trial met its primary endpoints, and in April 2019 SanBio received a Sakigake Designation for SB623, which is a Japanese version of a fast track to approval.
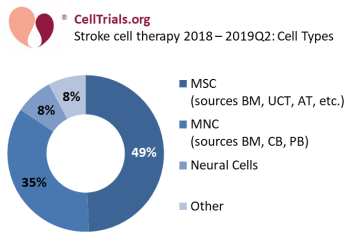 The most common cell type used in stroke cell therapy is mesenchymal stem/stromal cells (MSC), and while they are sometimes called by other names in company products, nonetheless they comprise 49% of these 77 trials (see Figure 2: Stroke cell therapy - cell types). The other common group is mononuclear cells (MNC) from a blood-based source comprise 35% of these trials. Stroke therapy with cord blood MNC make up 17% of the total trials.
The most common cell type used in stroke cell therapy is mesenchymal stem/stromal cells (MSC), and while they are sometimes called by other names in company products, nonetheless they comprise 49% of these 77 trials (see Figure 2: Stroke cell therapy - cell types). The other common group is mononuclear cells (MNC) from a blood-based source comprise 35% of these trials. Stroke therapy with cord blood MNC make up 17% of the total trials.
Cord blood is emerging as a serious competitor in cell therapy for stroke. The main reason is because MNC from cord blood trigger less graft-versus–host reaction than adult sources of MNC. Since 2011, every single stroke trial that sourced MNC from bone marrow or peripheral blood relied on autologous cells, where the patient had to undergo a harvest of his or her own cells. By comparison, all of the adult stroke trials that utilize cord blood are allogeneic (exceptions are a couple of trials designed to treat stroke in infants).
A recent trend is for stroke therapy with cord blood cells to push against the envelope of HLA matching. In March 2015, Duke University embarked on a novel phase 1 trial NCT02397018 to test the safety of treating stroke patients with an infusion of cord blood that was completely unmatched except for standard ABO blood typing. Ten patients between ages 45 and 79 that had suffered an ischemic stroke received intravenous infusions within 3 to 10 days after the stroke, and no adverse events related to the therapy were noted. The results from this trial were published in May 20189. Currently, Duke and collaborators are running a larger phase 2 double-blind trial NCT03004976 at 6 medical centers with the target enrollment of treating 100 patients by March 2020.
There are other notable examples of cord blood trials for stroke. The hybrid cord blood bank StemCyte has supported trials NCT01673932 in Hong Kong and NCT02433509 in Taiwan that treated patients with allogeneic cord blood having a minimum 4 out of 6 HLA match. In South Korea, the research center at Bundang CHA Hospital ran trial NCT01884155 in 2013, and in July 2019 they registered a phase 2 trial NCT04013646 that will treat stroke patients with allogeneic cord blood having a minimum 3 out of 6 HLA match. Also in July 2019, the for-profit clinic Blue Horizon International posted ISRCTN10678357 on the WHO trial registry, stating that they had a registry of 97 stroke patients treated in China with cord blood that only had ABO blood type match.
If clinical trials of allogeneic cord blood therapy for stroke continue to meet their endpoints, this could be an exciting new application for donated cord blood. In the United States, about 795,000 people suffer a stroke each year, and 140,000 are fatal1-3. If only 1% of these patients received cell therapy, that would be comparable to the total number of allogeneic stem cell transplants per year in the United States10.
Ultimately, a successful cord blood therapy will find itself in competition against cell therapy products for stroke that are already near approval. The possibility to utilize cord blood cells as an “off-the-shelf” product (actually out of the cryogenic freezer) with no HLA matching would make cord blood more competitive against other cell therapies that are based on MSC and operate as universal donor products.
Regardless of how the research on allogeneic cord blood for stroke evolves, it promises to be an exciting topic to follow.
References
- American Stroke Association Stroke.org
- Mozaffarian D, Benjamin EJ, Go AS et al. Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation 2015; 131: e29– e322.
- Feigin VL, Forouzanfar MH, Krishnamurthi R et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010; Lancet 2014; 383: 245– 254.
- Kapinos KA, Briscombe B, Gračner T, Strong A, Whaley C, Hoch E, Hlávka JP, Case SR, Chen PG. Challenges to the Sustainability of the U.S. Public Cord Blood System. 2017; RAND Corporation
- Verter F, Mandot VA. Public Banking Without Bankrupting. July 2019; Parent's Guide to Cord Blood Newsletter
- Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): Bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 2009; 40(2):510–515.
- Kalladka D, Muir KW. Brain repair: Cell therapy in stroke. Stem Cells Cloning 2014; 7:31– 44.
- Cell Trials Data. Accessed Sept 2019; CellTrials.org
- Laskowitz DT, Bennett ER, Durham RJ, Volpi JJ, Wiese JR, Frankel M, Shpall E, Wilson JM, Troy J, Kurtzberg J. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Translational Medicine 2018; 7(7):521-529
- Center for International Blood and Marrow Transplant Research (CIBMTR). Number of HCTs performed in the United States and reported to CIBMTR - by year and donor type 2012-2016. Data


