Jste zde
Are we ready for placenta cells to enter the clinic?
Perinatal cells have been gaining impact in the field of regenerative medicine for over a decade1. In addition to the well-known hematopoietic stem cells from cord blood, other cells with stem/progenitor properties can be isolated from the human term placenta.
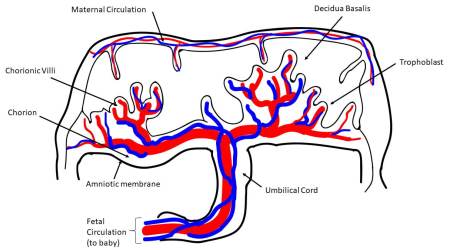 More specifically, different stem cells can be isolated from four major regions of the placenta: amniotic epithelial, amniotic mesenchymal stromal, chorionic mesenchymal stromal, and chorionic trophoblastic tissues, as established by the First International Workshop on Placenta-Derived Stem Cells2. Cells with characteristics of mesenchymal stromal/stem cells (MSC) can also be isolated from different placental regions, such as chorionic villi3-6, from the umbilical cord7-9, and also from the maternal component of placenta, known as the decidua10. Perinatal cells from the human amniotic fluid have also gained much attention in the field of regenerative medicine11.
More specifically, different stem cells can be isolated from four major regions of the placenta: amniotic epithelial, amniotic mesenchymal stromal, chorionic mesenchymal stromal, and chorionic trophoblastic tissues, as established by the First International Workshop on Placenta-Derived Stem Cells2. Cells with characteristics of mesenchymal stromal/stem cells (MSC) can also be isolated from different placental regions, such as chorionic villi3-6, from the umbilical cord7-9, and also from the maternal component of placenta, known as the decidua10. Perinatal cells from the human amniotic fluid have also gained much attention in the field of regenerative medicine11.
There are several reasons for the increased recognition of perinatal cells. First, the use of human term placenta lacks ethical concerns since it is generally regarded as biological waste, and there are also few costs associated to obtaining the placenta after birth. Second, it is easy to isolate cells from human term placenta. Moreover, of utmost importance is that placental MSC lack expression of human leukocyte antigens class II and other co-stimulatory molecules, and have intrinsic, potent immunomodulatory properties12-14, making them very attractive for transplantation in allogeneic settings. In fact, the intrinsic immunomodulatory properties of perinatal cells seem higher than that compared to other cells used in regenerative medicine (e.g. bone marrow MSC)15-17, thus substantiating their suitability for therapeutic approaches in regenerative medicine.
 There is a lot of research to understand how perinatal cells contribute to the regeneration of damaged tissues. Initially it was thought that the cells differentiated into tissue-specific cell types and engrafted in order to replace damaged tissues. A more recent and more widely-documented mechanism is that perinatal cells act via paracrine signaling through the release of bioactive mediators18,19. The paracrine signals induce regeneration by stimulating resident target cells to proliferate, and/or by inducing resident progenitor cells to differentiate. This mechanism has opened a new era in regenerative medicine.
There is a lot of research to understand how perinatal cells contribute to the regeneration of damaged tissues. Initially it was thought that the cells differentiated into tissue-specific cell types and engrafted in order to replace damaged tissues. A more recent and more widely-documented mechanism is that perinatal cells act via paracrine signaling through the release of bioactive mediators18,19. The paracrine signals induce regeneration by stimulating resident target cells to proliferate, and/or by inducing resident progenitor cells to differentiate. This mechanism has opened a new era in regenerative medicine.
To illustrate the issues that need further clarification, we focus on perinatal cells that have mesenchymal properties. The first important parameter to characterize these cells is the number of culture passages, or even better the population doubling time. Differences in the scale up of cell production between preclinical lab work versus human clinical trials could contribute to the failure of some therapies to transition from bench to bedside. Indications of population doubling time should be mandatory for defining the cell populations.
 Another parameter to consider for placenta-derived cells, as for MSC derived from other sources, is cell potency. Regulatory authorities have recently mandated the development of potency assays as part of the release criteria for advanced-phase clinical trials20. The well-established notions of sterility and cell viability, together with markers that identify MSC21, are the current accepted criteria for MSC release. However, the field of MSC therapy lacks functional-based assays for given disease indications, and defining robust and predictive markers and assays of potency remains a major issue in the cell-therapy field22.
Another parameter to consider for placenta-derived cells, as for MSC derived from other sources, is cell potency. Regulatory authorities have recently mandated the development of potency assays as part of the release criteria for advanced-phase clinical trials20. The well-established notions of sterility and cell viability, together with markers that identify MSC21, are the current accepted criteria for MSC release. However, the field of MSC therapy lacks functional-based assays for given disease indications, and defining robust and predictive markers and assays of potency remains a major issue in the cell-therapy field22.
The International Society for Cell Therapy (ISCT) has stressed the need to include immunophenotype and also immune-cell responder assays as an essential part of the release criteria for cells with immunomodulatory properties20. We consider this recommendation to be highly relevant to perinatal cells.
Another important parameter when considering cells from human placenta is contamination with trace amounts of maternal cells that can only de detected after several passages in culture. The consensus from the First International Workshop on Placenta Derived Stem Cells2 proposed the analysis of the fetal or maternal origin of cells. As mentioned previously, the placenta is a feto-maternal organ, and there are placental tissues where this could result in a mixed cell population. The cells should be characterized with highly sensitive techniques, such as quantitative PCR, not only immediately after cell isolation but also after cell culture.
To underline the need for caution, we reiterate our previous study which compared maternal cell contamination both after cell isolation and later after several culture passages23. We tested freshly isolated cell populations from the mesenchymal fraction of both the amnion and chorion regions of the placenta. In both cases the initial genomic PCR amplification did not detect maternal cells in the freshly isolated cell populations. Several passages later, maternal cells were detected in the populations isolated from the chorionic membrane, while cell populations from the amniotic membrane did not show presence of maternal alleles23.
References
- Couto, P.S., A. Bersenev, and F. Verter, The first decade of advanced cell therapy clinical trials using perinatal cells (2005-2015). Regen Med, 2017. 12(8): p. 953-968.
- Parolini, O., et al., Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells, 2008. 26(2): p. 300-11.
- Fukuchi, Y., et al., Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells, 2008. 22(5): p. 649-58.
- Igura, K., et al., Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy, 2004. 6(6): p. 543-53.
- Portmann-Lanz, C.B., et al., Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol, 2006. 194(3): p. 664-73.
- Castrechini, N.M., et al., Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta, 2010. 31(3): p. 203-12.
- Wang, H.S., et al., Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells, 2008. 22(7): p. 1330-7.
- Troyer, D.L. and M.L. Weiss, Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells, 2009. 26(3): p. 591-9.
- La Rocca, G., et al., Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol, 2009. 131(2): p. 267-82.
- in 't Anker, P.S., et al., Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells, 2008. 22(7): p. 1338-45.
- De Coppi, P., et al., Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol, 2007. 25(1): p. 100-6.
- Magatti, M., et al., Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells, 2009. 26(1): p. 182-92.
- Magatti, M., et al., Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant, 2009. 18(8): p. 899-914.
- Magatti, M., et al., Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J Tissue Eng Regen Med, 2016.
- Rossi, D., et al., Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One, 2012. 7(10): p. e46956.
- Moodley, Y., et al., Anti-inflammatory effects of adult stem cells in sustained lung injury: a comparative study. PLoS One, 2013. 8(8): p. e69299.
- Lee, J.M., et al., Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. International Immunopharmacology, 2012. 13(2): p. 219-224.
- Silini, A.R., et al., Is Immune Modulation the Mechanism Underlying the Beneficial Effects of Amniotic Cells and Their Derivatives in Regenerative Medicine? Cell Transplant, 2017. 26(4): p. 531-539.
- Silini, A., et al., Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther, 2013. 8(1): p. 6-14.
- de Wolf, C., M. van de Bovenkamp, and M. Hoefnagel, Regulatory perspective on in vitro potency assays for human mesenchymal stromal cells used in immunotherapy. Cytotherapy, 2017. 19(7): p. 784-797.
- Dominici, M., et al., Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006. 8(4): p. 315-7.
- Magatti, M., et al., The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin. Cell Transplant, 2018. 27(1): p. 31-44.
- Soncini, M., et al., Isolation and characterization of mesenchymal cells from human fetal membranes. J Tissue Eng Regen Med, 2007. 1(4): p. 296-305.


