Jste zde
Recruiting Clinical Trials of Cord Blood or Umbilical Cord Tissue
Half of family cord blood banks also offer storage of umbilical cord tissue. This was first established in our 2015 Cord Blood Industry Report1,2, and has since been widely quoted.
Whereas cord blood is a rich source of blood-forming (hematopoietic) stem cells known as HSC3, umbilical cord tissue is a rich source of mesenchymal stem/stromal cells known as MSC4-6. Cord blood is best known for its use in cord blood transplants7 to treat hematology and oncology disorders, but emerging therapies also use cord blood stem cells to treat brain injuries that occur in newborns, such as cerebral palsy8. The MSC in cord tissue are well known to suppress inflammation and auto-immune reactions9-11, and they can promote healing of muscle or bone injuries that are seen in cardiac or orthopedic medicine12-14.
Family banks encourage parents to store both cord blood and cord tissue, in order to have access to more forms of therapy in the future. But how many clinical trials are currently using these newborn stem cells in humans, what diagnoses are being treated, and where can families get access to these therapies?
Parent’s Guide to Cord Blood Foundation has set up two web pages which enable the public to search for currently recruiting clinical trials that use either cord blood or cord tissue. By the numbers, our search portals currently list 114 recruiting cord blood trials that are enrolling patients at 354 locations, and 49 recruiting umbilical cord tissue trials that are enrolling patients at 65 locations. Currently the cord blood data is dated March 2017 and the umbilical cord tissue data is dated Sept. 2017.
These portals only display trials that are recruiting new patients right now. The portals are primarily intended for use by the general public, and patients may be frustrated if they encounter trials with status of “ongoing but not recruiting” or “pending” and “not yet recruiting”. Our search portals cover a dozen clinical trial registries from around the world, not just ClinicalTrials.gov.
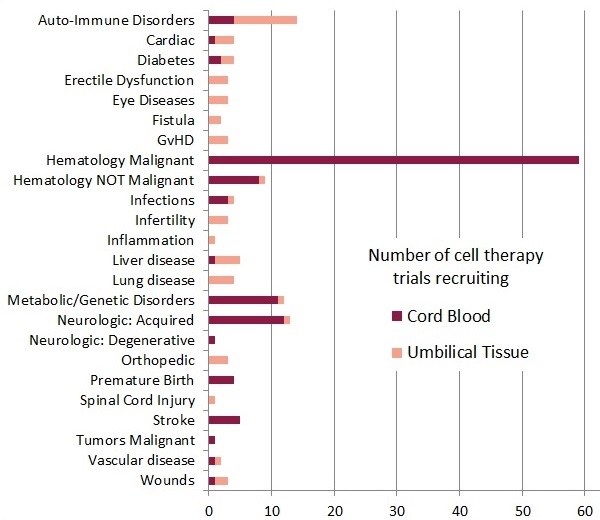
The accompanying bar diagram shows the number of trials enrolling, grouped by diagnosis category and color coded by cord blood or umbilical cord tissue. It is very important to understand that this diagram is a snapshot in time, showing the therapies that are available in trials right now. This does not represent all therapies that could possibly be offered with cord blood or umbilical cord tissue, and it does not include therapies that have been offered in past trials that have already closed. Also note that the diagnosis menu on the website provides a somewhat different list of names for conditions, simplified for users, and do not match the names used here.
Among the 114 open cord blood trials, 54 are traditional stem cell transplants, and 60 are advanced cell therapy, whereas the 49 cord tissue trials are all advanced cell therapy. Advanced cell therapy is defined15 by both the EMA and the FDA as all therapies in which cells are more than minimally manipulated, and/or their action is not homologous. Many authors use the phrase “novel therapies” to describe regenerative medicine and/or immunotherapy, but there is no community consensus as to what exactly constitutes a “novel” therapy. The term “advanced cell therapy” should be preferred, because it has a formal definition established by regulatory agencies.
If we restrict our comparison of the recruiting cord blood and umbilical cord tissue trials to only the advanced cell therapy trials, we get the second bar graph of trials per diagnosis group.
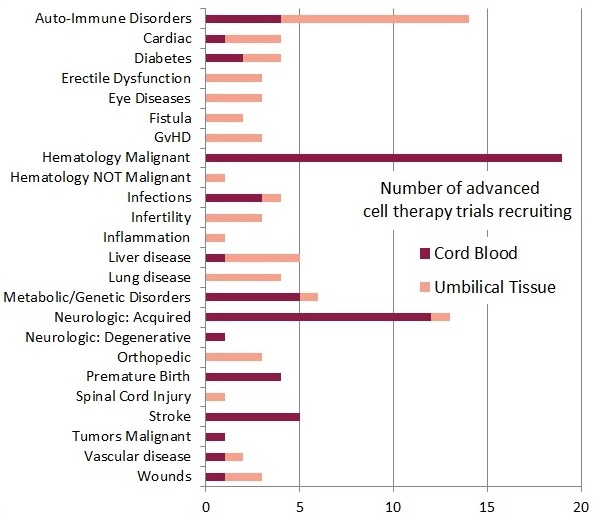
The top three types of therapy in this chart are as follows: First, the biggest group is 19 cord blood trials for hematologic malignancies that are advanced cell therapies because they use expanded cells or various forms of immunotherapy. Second, worldwide there are 18 cord blood trials and 1 cord tissue trial that are currently recruiting for neurologic conditions, including conditions acquired near birth, degenerative conditions of old age, and stroke. Third, 10 trials are currently using MSC from umbilical cord tissue to treat auto-immune disorders, including Amyotrophic Lateral Sclerosis, Lupus, Multiple Sclerosis, and Rheumatoid Arthritis.
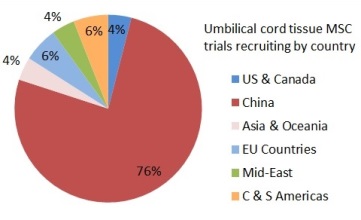
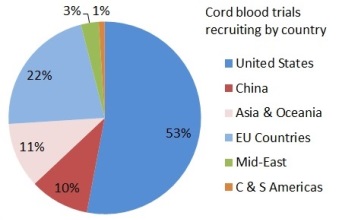 Depending on where they live, patients might find it easier or harder to get access to a clinical trial with newborn stem cells. The two pie charts show the number of trials that are enrolling per country, for either cord blood or umbilical cord tissue. In these pie charts we counted a trial once for each country where it enrolls, so that a few trials that are recruiting in multiple countries were counted multiple times. Specifically, the 114 cord blood trials are recruiting in 138 countries and the 49 umbilical cord tissue trials are recruiting in 49 countries. There are many cord blood trials in the United States and Western Europe, especially for hematology and oncology indications. By comparison, the vast majority of umbilical cord tissue trials can only be accessed in China.
Depending on where they live, patients might find it easier or harder to get access to a clinical trial with newborn stem cells. The two pie charts show the number of trials that are enrolling per country, for either cord blood or umbilical cord tissue. In these pie charts we counted a trial once for each country where it enrolls, so that a few trials that are recruiting in multiple countries were counted multiple times. Specifically, the 114 cord blood trials are recruiting in 138 countries and the 49 umbilical cord tissue trials are recruiting in 49 countries. There are many cord blood trials in the United States and Western Europe, especially for hematology and oncology indications. By comparison, the vast majority of umbilical cord tissue trials can only be accessed in China.
 Another concern to patients and parents is whether the recruiting trials require the family to have their own newborn stem cells in storage. At present, 100% of the recruiting trials with MSC from umbilical cord tissue are sourcing the cells from umbilical cords that were donated at birth, so these trials are available to any patient. In the case of cord blood trials, all of the traditional stem cell transplants are using donated cord blood from public banks. However 13 of the 60 advanced cell therapy trials require that the patient has stored their own (autologous) cord blood. Nine of these autologous trials are for cerebral palsy and neonatal encephalopathy, and they are taking place in 6 countries around the world. The rest of the 19 trials that are currently recruiting for neurologic conditions do not require autologous newborn stem cells.
Another concern to patients and parents is whether the recruiting trials require the family to have their own newborn stem cells in storage. At present, 100% of the recruiting trials with MSC from umbilical cord tissue are sourcing the cells from umbilical cords that were donated at birth, so these trials are available to any patient. In the case of cord blood trials, all of the traditional stem cell transplants are using donated cord blood from public banks. However 13 of the 60 advanced cell therapy trials require that the patient has stored their own (autologous) cord blood. Nine of these autologous trials are for cerebral palsy and neonatal encephalopathy, and they are taking place in 6 countries around the world. The rest of the 19 trials that are currently recruiting for neurologic conditions do not require autologous newborn stem cells.
This is an exciting time for both patient families and professionals, as more trials are opening up around the world that will perform advanced cell therapy with newborn stem cells. We hope to keep the community appraised with periodic updates that review the changing contents of our trials portals and the evolving statistics of the trials.
References
- Cord Blood Industry Report 2015; accessed from http://cordbloodindustryreport.org
- Couto PS, Verter F. Survey of How Cord Blood Banks Process Cord Tissue Transfusion. Poster Abstract 2016 International Cord Blood Symposium. Transfusion 2016; 56(6):A3-A4
- Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 1989; 86(10):3828–32
- Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human Umbilical Cord Perivascular (HUCPV) Cells: A Source of Mesenchymal Progenitors. Stem Cells. 2005; 23(2), 220–229
- Secco M, Zucconi E, Vieira, Fogaca LLQ, Cerqueira A, Carvalho MDF, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008; 26(1):146–50
- Schugar RC, Chirieleison SM, Wescoe KE, Schmidt BT, Askew Y, Nance JJ, Evron JM, Peault B, Deasy BM. High Harvest Yield, High Expansion, and Phenotype Stability of CD146 Mesenchymal Stromal Cells from Whole Primitive Human Umbilical Cord Tissue. J. Biomedicine Biotechnology. 2009; 2009:1–11
- Ballen KK, Verter F, Kurtzberg J. Umbilical cord blood donation: public or private? Bone Marrow Transplantation. 2015; 50(10):1271–1278
- Sun J. Emerging use of cord blood in regenerative medicine. Cell Gene Therapy Insights 2017; 3(7):573-581
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005; 105(4):1815-22
- Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annual Review Pathology. 2011; 6:457-78.
- Bersenev A, Couto, PS, Verter F. Cell Therapy Clinical Trials for AutoImmune Diagnoses 2011-2015. 2016; Parent's Guide to Cord Blood Foundation newsletter April 2016
- Sanina C, Hare JM. Mesenchymal Stem Cells as a Biological Drug for Heart Disease. Circulation Research. 2015; 117:229-233
- Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015). Stem Cell Research & Therapy 2016; 7:82
- Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. International Orthopaedics 2016; 40(8):1755-1765
- CellTrials.org Definitions 2017; accessed from https://celltrials.org/definitions


