Jste zde
Amniotic Membrane uses in Ophthalmology
Amniotic membrane consists of a combination of tissue and cells, which when used as a biological dressing, helps wound healing by acting as a foundation for re-growth of soft tissue. Biologically active cells in the epithelial and stromal layers of the amnion deliver growth factors and cytokines with anti-inflammatory, anti-bacterial, anti-immunogenic and anti-fibrotic properties1.
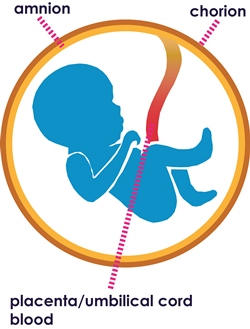 The human placental membranes were first used in the early 1900’s as a skin substitute. These procedures included the amniotic and chorionic membranes. The amniotic membrane was first used on its own in ophthalmology in 1940, when De Rotth suggested it for the use in the treatment of an ocular burn wound.2 The treatment was successful, but the use of amniotic membrane then went out of favour until the early 1990’s, when improved processing and storage techniques were developed.
The human placental membranes were first used in the early 1900’s as a skin substitute. These procedures included the amniotic and chorionic membranes. The amniotic membrane was first used on its own in ophthalmology in 1940, when De Rotth suggested it for the use in the treatment of an ocular burn wound.2 The treatment was successful, but the use of amniotic membrane then went out of favour until the early 1990’s, when improved processing and storage techniques were developed.
Over the past 25 years, literally thousands of ocular surgical procedures have used amniotic membranes to treat various ocular disorders and more than 700 peer reviewed articles have been published. Amniotic membrane has also been used in various reconstructive surgical procedures as a biological dressing. These procedures include: biological bandages for burns and non-healing diabetic and venous ulcers, repair of abdominal hernia, closure of the pericardium, and as a barrier to the prevention of surgical adhesions.3,4
In utero, the amniotic membrane develops from the extra-embryonic tissue including both foetal and maternal components which are kept together by the chorionic villi. The foetal component consists of the amniotic and chorionic foetal membranes, which separates the foetus from the endometrium4. The amniotic membrane (AM) is the innermost layer of the foetal membranes of the placenta. It is avascular and has an epithelial layer with a sub-adjacent avascular stromal layer5,6.
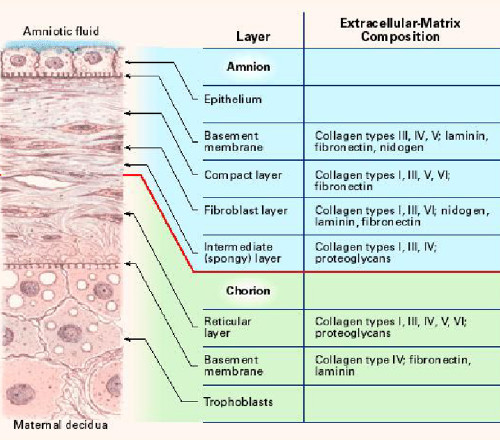 The amniotic membrane is one of the thickest membranes in the human body. The adjacent illustration4 displays a cross section of the human amniotic membrane indicating the biochemical characteristics of its five layers7:
The amniotic membrane is one of the thickest membranes in the human body. The adjacent illustration4 displays a cross section of the human amniotic membrane indicating the biochemical characteristics of its five layers7:
- cuboidal epithelium layer;
- basement membrane;
- compact layer;
- fibroblast layer; and
- intermediate (spongy) layer.
How the Amniotic Membrane influences healing
The basement membrane of the amniotic membrane is a thin layer composed of reticular fibres, closely adherent to the amniotic epithelium, while the adjacent compact layer is more dense and devoid of cells consisting of a complex reticular network.3,4 It has low immunogenic potential and contains bioactive factors that have been shown to be beneficial in wound treatment, such as: collagen, cell-adhesion bio-active factors (fibronectin, laminins, proteoglycans and glycosaminoglycans) and growth factors.3,7
Therapeutic benefits that can be derived from the biological factors and cytokines in amniotic membranes:
- Anti-inflammatory effects - Amniotic membrane suppress the pro-inflammatory cytokines.
- Anti-microbial - The amniotic membrane serves as a physical barrier against the external environment with close adherence of the membrane to the wound surface, as well as producing anti-microbial cytokines.
- Anti-scarring and Anti-adhesive activity - Amniotic membrane reduces protease activity via the secretion of TIMP’s (tissue inhibitors of metalloproteinases) and therefore has an anti-fibrotic effect. There is also down regulation of TGF-ß which is responsible for the activation of fibroblasts and prevents the adhesion of injured surfaces to each other.
- Non-immunogenic and low antigenicity – Amnion has low expression of histocompatibility (HLA Class II) antigens A, B, C DR or beta2 microglobulin, and can therefore be used as an allogeneic dressing without any problem.
- Analgesic properties – There is rapid pain relief due to efficient covering of the nerve endings.
- Anti-angiogenic - Prevents the formation of new blood vessels.
- Contains collagen types IV, V and VII - These promote cellular differentiation and adhesion.
- Promotion of Epithelialisation - The basement membrane is a good substrate for cell migration and the maintenance of epithelial cell polarity. Together with the expression of growth factors such as KGF, b-FGF, HGF and TGF-ß, there is promotion of epithelialisation, cell proliferation and differentiation.
In overview, the amniotic membrane mechanism of action relies on its high concentrations of cytokines and growth factors. The use of the amniotic membrane to cover inflamed or exposed areas favourably influences the wound healing process, as well as reducing the levels of pain and discomfort of the patient.3
The pragmatic aspect of using the thick amniotic membrane is that it conforms easily to the wound surface and can be either glued or sutured to the wound surface. The membrane is hydrophilic and naturally absorbs the surrounding fluids. As part of the healing process, amniotic membrane resorbs into the wound.
Amniotic membrane is widely used in eye surgery as a biological bandage to heal or replace damaged eye tissue. It is thin, lightweight, elastic and almost transparent making it suitable for use on the surface of the eye. It can be sutured or glued into place with tissue glue. Amniotic membrane is used to treat chemical burns, ulcers of the cornea or conjunctiva, diseases causing devastating ulcerations and during surgical procedures where eye tissue has to be excised.
The examples below showcase three applications in ophthalmology where amniotic membrane is used successfully.
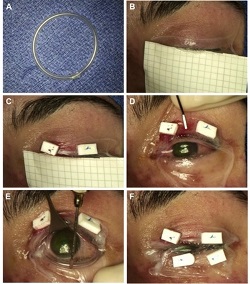
This is a rare, acute, blistering disease of the skin and mucous membranes caused by an immune reaction triggered by drugs or infections. The clinical picture is similar to partial-thickness burn wounds and eye involvement is very common. Amniotic membrane is used successfully to speed up healing and alleviate pain.
Example 1 Figure: Amniotic membrane (AM) transplantation utilizing a single 5 x10 cm sheet. (A) Creation of symblepheron ring with intravenous tubing. (B) Placement of AM over upper eyelid. (C) Anchoring of AM using 6-0 polypropylene mattress sutures and bolsters. (D) Unfolding of AM over the ocular surface. (E) Placement of the custom-made symblepheron ring in the fornices. The ring is already pushed into the upper fornix and is being gently deposited into the lower fornix. (F) Anchoring of AM to lower eyelid.
Photo taken from: Ma K N , Thanos A, Chodosh J, Shah A S, Mantagos I S; A Novel Technique for Amniotic Membrane Transplantation in patients with Acute Stevens-Johnson Syndrome; The Ocular Surface, 2016; 14(1):31-36.
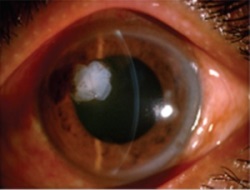
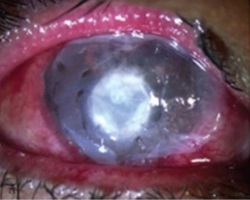
These are healed effectively and quickly by using amniotic membrane as a biological dressing to fill the cavity.
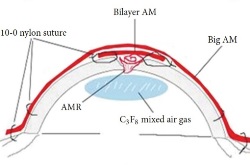
Example 2 Figure: Surgical steps for corneal perforation surgery. For a smaller ulcer and perforation with an abrupt edge, a roll of amniotic membrane (AM) is pushed into the perforation and fixed with cross-stitch fixation, as shown in the top image.
For the larger perforation in the middle image, first, AM was folded into a roll and plugged into the perforation. Second, a bilayer AM was covered by the roll and ulcer with the epithelial side up and secured with 10-0 nylon sutures. Third, 0.3mL of 20% C3F8 (perfluoropropane) was injected into the anterior chamber. Finally, a larger piece of AM was applied over the entire cornea as a temporary patch and anchored with 2 laps running 10-0 nylon sutures to the corneal limbus and perilimbal episclera.
The schematic in the bottom image ilustrates the placement of the amniotic membrane roll, the bilayer amniotic membrane, and the big piece of amniotic membrane that is applied over the entire cornea.
Photos taken from: Fan J, Wang M, Zhong F; Improvement of Amniotic Membrane Method for the Treatment of Corneal Perforation; BioMed Research International; 2016; Article ID 1693815.
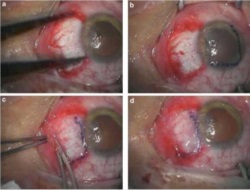
Amniotic membrane is used successfully in this procedure which is commonly performed. Pterygium is a benign growth on the conjunctiva of the eye, usually in the area closest to the nose. These growths can cause irritation in the eye and need to be removed surgically. The deficit left after the removal is successfully healed with amniotic membrane placements as seen below.
Example 3 Figure: Surgical procedures of multi-layer Amniotic Membrane Graft. (a) The bare scleral defect was measured with caliper after the excision of the pterygium. (b) Amniotic membrane was marked with gentian violet to ensure that a proper-sized graft, which would cover the defect area, was obtained. The membrane was placed over the cornea with the epithelial/basement membrane side on top. (c) The membrane was slid over the scleral bed and the edges were ‘pinched’ together with the recipient conjunctiva using a smooth forceps. (d) The stability of the membrane was checked with a cellulose sponge.
Photos taken from: Fahmy RM; Pterygium Resection with Amniotic Membrane Grafting in a Patient with Xeroderma Pigmentosum; Austin J Clin Ophthalmol; 2016 Vol 3 (2); ISSN : 2381-9162
In summary, Amniotic membrane has been shown to be an ideal biological dressing in ophthalmology and is being used worldwide. Its properties are ideal to promote healing and afford comfort to the patient.
References
- Perepelkin N, Hayward K, Mokeona T et al.; Cryopreserved amniotic membrane as a transplant allograft: viability and post-transplant outcome; Cell and Tissue Banking 2016; 17(1):39–50.
- Rahman I, Said DG, Maharajan VS, Dua HS. Amniotic membrane in ophthalmology: indications and limitations. Eye. 2009; 23(10):1954–61.
- Dua HS, Gomes JA., King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Survey of Ophthalmology. 2004; 49(1):51–77.
- Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. European Cells and Materials. 2008; 15:88–99.
- Tyszkiewicz JT, Uhrynowska-Tyszkiewicz IA, Kaminski A, Dziedzic-Goclawska A. Amnion allografts prepared in the Central Tissue Bank in Warsaw. Annals of transplantation: quarterly of the Polish Transplantation Society. 1999; 4(3-4):85.
- Bourne G. The Fœtal Membranes. A Review of the Anatomy of Normal Amnion and Chorion and Some Aspects of Their Function. Postgrad Med J. 1962; 38(438):193–201.
- Jiang A, Li C, Gao Y, Zhang M, Hu J, Kuang W, et al. In vivo and in vitro inhibitory effect of amniotic extraction on neovascularization. Cornea. 2006; 25:S36–S40.


