Jste zde
Cord Blood as starting material for Induced Pluripotent Stem Cells

Cord blood banking, both public and private, has illustrated the power of having needed stem cells available for potential use. It is clear that stem cells that are identical to your own are the best, followed by those from near relatives, followed by those from donors who carry the same HLA phenotype. By best I mean that the body does not initiate a process of rejection against them.
The reason why unrelated donors are not as good as your own cells, even when they are HLA matched, is simply because through evolution nature has devised elaborate methods to distinguish self from non-self. The major HLA types are only one of the methods that nature uses. There are also minor HLA antigens, and a class of immune response termed innate immunity. Just as nature has evolved methods to differentiate self from non-self, nature has also evolved tolerance mechanisms, such as when a mother spends nine months pregnant with a baby that has a different HLA type.
As we humans have developed a better understanding of the immune system and rejection responses, we have been able to develop drugs to suppress the rejection response when we perform organ transplants or stem cell transplants. However, when drugs are used to fool the immune system into accepting a transplant, the patients must stay on the drugs for the rest of their lives, and this leads to many long term side effects.
Recently another type of stem cell has been manufactured by scientists. The invention of induced Pluripotent Stem Cells (iPSC) was honored by a Nobel Prize in 2012 to Dr. Shinya Yamanaka and Dr. John Gurdon whose work Dr. Yamanaka built upon. They showed that it was possible to take any cell from the body and, with a few gene engineering tricks, convert it into a stem cell. The iPSC are more like embryonic stem cells from the earliest stage of fetal development than like cord blood stem cells. The iPSC can make all the lineages of cells in a body.
Scientists do not imagine that iPSC will replace cord blood for therapeutic purposes. Making iPSC is not as cost effective as simply banking cord blood. Also the gene engineering involved in the manufacture of iPSC creates additional challenges to their regulatory approval for clinical applications.
Rather scientists imagine that cord blood banks could serve as the scientific and business model for how iPSC could be banked and distributed. Equally important, scientists have shown that cord blood cells are a very good starting material for making iPSC. Cord blood stem cells are immunologically pristine, are collected in the correct manner for a cell therapy starting material, have regulatory approval for many indications, and in the public cord blood banks they are already HLA typed.
Therapy models that scientists and clinicians are proposing for iPSC-based treatments are summarized in the figure below. In the middle column named One to Many, one batch of iPSC can be made, cryopreserved, and used to treat hundreds of thousands of people. While patients would have to take immune suppression to prevent rejection, the cost of making the cells and distributing them would be cheap when done on a mass scale. Scientists also hope that we may discover additional less costly methods to suppress the immune system.
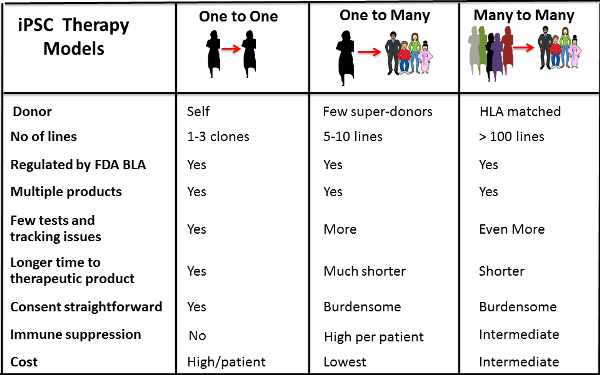
Other scientists have suggested that in parallel we could develop a Many to Many treatment model by making a few lines of super-donor iPS which can be used to treat multiple individuals because they have ancestral HLA types that do not trigger strong rejection reactions. This is analogous to banking a blood transfusion from a donor who has blood type O-negative, which can be tolerated by patients of all blood types. Scientists estimate that as few as 200 super-donor iPSC lines may be sufficient to treat most individuals.
The third One to One strategy is akin to how family cord blood banks operate already. Collect some of your own stem cells and bank them, plus make iPSC from them for companion storage. These personalized iPSC would be the best match for yourself and could be used as a self-renewable source of multiple cell types that you may need at different times in your life.
The ability of cord blood banks to participate in this exciting new field has important implications for people choosing to store cord blood. The amount of cord blood collected at birth is no longer a limitation. If a small portion of the cord blood was used to make iPSC, while the bulk was banked as usual, then the additional cells generated on the iPSC pathway could provide for multiple additional therapies from the original cord blood donor. Moreover, should they choose, a donor could provide iPSC cells to others without depleting the cells they needed for themselves.
The combination of cord blood plus iPSC banking is a win-win for all based on the larger dividing capacity of the iPSC cell. Cord blood banks could and should develop a strategy to provide this exciting option to their clients. Parents should ask family cord blood banks, what is their ability to make and store iPSC from cord blood?