当前位置
Therapeutic MSC Infusions: When more of a good thing is not always better
Mesenchymal stromal/stem cell (MSC) therapies are very popular in regenerative medicine5,6. They have been studied for decades in clinical trials treating a multitude of conditions7-9. So far, none of these therapies have been approved by the United States FDA for use outside of clinical trials, although there are first MSC products with approvals in Canada, Japan, and New Zealand. Nevertheless, within the United States alone there are hundreds of commercial clinics that are selling MSC therapies to consumers, and many families from the United States travel to other countries to purchase MSC therapeutics10.
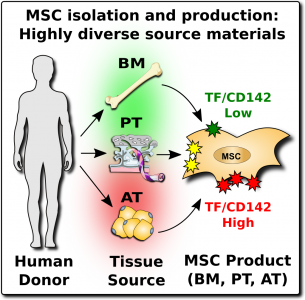 Consumers of MSC therapeutics, both in official trials and at unofficial clinics, should be aware that MSCs are a heterogeneous product1-6,11-13. They are heterogeneous for several reasons: (1) because MSCs from different parts of the human body have different native properties, and (2) because MSCs from the same tissue source will vary widely between donors, and (3) because the laboratory steps to isolate and culture MSCs introduce additional variations.
Consumers of MSC therapeutics, both in official trials and at unofficial clinics, should be aware that MSCs are a heterogeneous product1-6,11-13. They are heterogeneous for several reasons: (1) because MSCs from different parts of the human body have different native properties, and (2) because MSCs from the same tissue source will vary widely between donors, and (3) because the laboratory steps to isolate and culture MSCs introduce additional variations.
A potential hazard of MSC therapeutics is that when they come into contact with blood they can trigger the instant blood-mediated inflammatory reaction, also known as IBMIR1-4. This blood incompatibility reaction can cause a clot, or thromboembolism, to form in the patient’s blood stream. According to the World Health Organization, blood clots are one of the most frequent causes of death worldwide14. Blood clots can lead to heart attacks and strokes, which are the top two causes of death, accounting for about 25% of the world’s total deaths.
Infusions of MSC therapeutics that are quality controlled and given at low doses have proven very safe in clinical trials15-17. However, there are case reports of severe adverse events among patients that visited commercial clinics where they received MSC products under conditions with less testing and supervision1-4,18-20. Because MSC therapeutics are heterogeneous, the risk of a complication varies depending on the source of the MSCs and how they were processed.
We have identified a specific assay which can help assess the risk of clotting during or after infusions of MSC therapeutics. In particular, the MSC cellular product or exosome product derived from MSC should be tested for levels of procoagulant tissue factor (TF/CD142) prior to clinical administration. In addition, both patients and providers should be trained to be more aware of the symptoms of thromboembolism, and the necessary interventions21.
The first descriptions of MSCs identified them as multipotent mesenchymal precursor cells found in bone marrow that can undergo differentiation into characteristic tissue lineages: cartilage, bone, and fat22,23. There is still some debate about whether MSC should be called stem cells or stromal cells. We have learned that MSCs are beneficial for many medical conditions because they modulate the immune system (“immunomodulatory”) and promote healing (“regenerative”)5,6.
 When MSC therapeutics are given to a patient, they are not expected to engraft and persist in the patient’s body. The expectation is that the MSCs will suppress inflammation and promote natural healing mechanisms, but the MSCs themselves will die off and disappear. Currently, MSCs have potential therapeutic value for many clinical indications where there is an unmet need24.
When MSC therapeutics are given to a patient, they are not expected to engraft and persist in the patient’s body. The expectation is that the MSCs will suppress inflammation and promote natural healing mechanisms, but the MSCs themselves will die off and disappear. Currently, MSCs have potential therapeutic value for many clinical indications where there is an unmet need24.
Over the years, MSC infusions have been given to thousands of patients in clinical trials. Several reviews and meta-analyses have established that MSC infusions are very safe when properly administered15-17. So far, around two dozen studies looked at the incidence of blood clots during controlled clinical trials, and they found 31 events among 1112 patients, which is a 3% incidence17. Based on this professional literature, it has become almost an article of faith among clinicians that it is very safe to give MSC infusions to patients.
However, a number of case reports have described patients that developed severe thromboembolisms after MSC infusions at commercial clinics, and some of those patients died1-4,18-20. Given the worldwide prevalence of patients seeking MSC therapeutics at unofficial clinics, it is important to understand the safety limits and practical guidelines for MSC infusions.
After MSCs were first discovered in bone marrow, many of the early clinical trials with MSCs relied on bone marrow as the cell source. But researchers later learned that MSCs are a perivascular cell type that occurs near the blood vessels in many tissues of the body25. This has led to a large diversification of MSC products. During the past decade, the sources of MSCs in clinical trials have been divided primarily between bone marrow, adipose (fat) tissue, and perinatal tissues such as the umbilical cord and the placenta7-9. Since 2016, perinatal sources have been the biggest category each year9. The methods of growing MSCs in the laboratory have also evolved over time. Nowadays, pharmaceutical companies that produce MSC therapeutics often rely on bioreactors to grow batches of cells with uniform characteristics26.
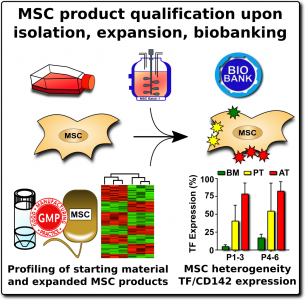
When researchers produce MSCs for scientific studies and clinical programs, they rely on a set of ‘minimal criteria’ to define MSC that were established in 200627. The three primary elements of the minimal criteria are: (1) The cultured cells display an adherent morphology (they form a characteristic cell layer that sticks to the culture dish), (2) Flow cytometry of the cells demonstrates that they express a set of agreed upon biomarkers, and (3) The cells demonstrate that they can differentiate into MSC lineages such as cartilage , bone, and fat cells.
By comparison, when MSCs are isolated and cultured in unofficial clinics, they may not undergo any validation against these minimal criteria. The cost of a high-quality flow cytometer is near $100K, so small laboratories do not own one. Some commercial clinics will take pieces of umbilical cord tissue, plate them in culture dishes, and assume that any adherent cell population which grows out must be MSCs.
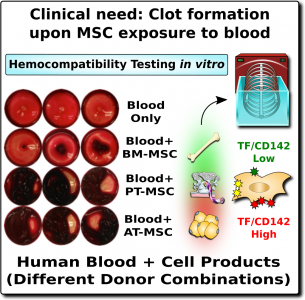 The word “hemocompatibility” describes whether a medical device or drug is compatible with blood and therefore safe for infusion. Hemocompatibility is of such importance for patient safety, that is has been strictly regulated for decades (e.g., ISO-10993-4)1,28. Just as a blood clot can be very dangerous, any drugs that inhibit clotting are also dangerous because they can trigger internal bleeding. Thus, in medicine, both clotting and bleeding are a double-edged sword that need to be handled with great caution.
The word “hemocompatibility” describes whether a medical device or drug is compatible with blood and therefore safe for infusion. Hemocompatibility is of such importance for patient safety, that is has been strictly regulated for decades (e.g., ISO-10993-4)1,28. Just as a blood clot can be very dangerous, any drugs that inhibit clotting are also dangerous because they can trigger internal bleeding. Thus, in medicine, both clotting and bleeding are a double-edged sword that need to be handled with great caution.
In the case of MSC therapeutics, hemocompatibility mainly depends on their expression of a highly procoagulant molecule called tissue factor (TF/CD142)1-4. This molecule is an extremely potent trigger of the TF-pathway of coagulation, which initiates a clotting or thrombotic response once in contact with blood. Since MSCs originate in perivascular zones of the body surrounding blood vessels, it stands to reason that MSCs can play a role in maintaining vascular integrity by promoting clotting after blood vessel injury1. Only minute amounts of this procoagulant molecule from MSC can elicit strong thrombotic responses in the vasculature upon cell infusion.
The expression of this procoagulant tissue factor (TF/CD142) is a feature that varies greatly between different sources of MSCs1-4. The MSCs in bone marrow (BM-MSC) reside next to the stem cells that make blood, and they are very compatible with blood. Hence it is no surprise that the initial safety studies on BM-MSCs showed that they are very safe to deliver by infusion. But other perivascular sources of MSCs, such as perinatal tissue (PT-MSC) or fat (AT-MSC), express much higher levels of procoagulant TF/CD1421-4. Even when the therapeutic product is exosomes derived from MSC, they can carry TF/CD142, because it is one of the membrane proteins expressed by their parent cells. Due to these variations, hemocompatibility at high doses varies across different MSC therapeutics1-4.
The typical doses of MSC infusions in clinical trials range from 1 to 10 million cells per kilogram of body weight of the treated patient2,7. Thus, for a person weighing 80 kilograms (160 pounds) this would be 80-800 million cells per infusion.
Unfortunately, it is not possible to simply set a number and say “don’t go over this MSC dose”. There are three reasons for this. First, there is a very high degree of variation among MSC therapeutics in their content of procoagulant tissue factor (TF/CD142). Second, what is even more important, there is a high degree of variation in patient reaction to procoagulant tissue factor (TF/CD142). Some patients are at particularly high risk due to their underlying medical conditions or a familial predisposition to clotting19. Third, at this time we don’t know if children have more or less risk than the 3% rate of thrombotic events seen in adult clinical trials of MSCs. Given these three issues, for now the best way to approach the small but not negligible risk of thromboembolism is to establish better safety protocols around the testing of patients and products, before and after therapy1-3,13.
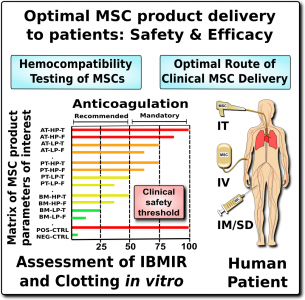 We recommend that all MSC therapeutics in clinical use should be tested for their expression of the highly procoagulant tissue factor (TF/CD142)1. This does not have to be a complicated or expensive test. Advanced laboratories can assess the amount of TF/CD142 on the surface of MSCs with flow cytometry, although a control sample will be needed to set reference values. A less expensive test can be performed on the supernatant or cell lysate of the MSC cultures using validated ELISA or Western blot testing. We encourage more researchers to characterize and publish the TF/CD142 content of their MSC therapeutics to build a community database.
We recommend that all MSC therapeutics in clinical use should be tested for their expression of the highly procoagulant tissue factor (TF/CD142)1. This does not have to be a complicated or expensive test. Advanced laboratories can assess the amount of TF/CD142 on the surface of MSCs with flow cytometry, although a control sample will be needed to set reference values. A less expensive test can be performed on the supernatant or cell lysate of the MSC cultures using validated ELISA or Western blot testing. We encourage more researchers to characterize and publish the TF/CD142 content of their MSC therapeutics to build a community database.
Patients, as well as their caregivers and medical providers, need to be aware of the signs and symptoms of thromboembolism21. The symptoms of a thromboembolism may not occur until hours or days after the infusion. And, most importantly, a thromboembolism cannot be managed properly in an outpatient clinic. The typical treatment for a thromboembolism is to give the patient anti-clotting drugs intravenously. However, these drugs carry a risk of triggering hemorrhage, so it would be irresponsible to give them to a patient without first confirming there is a clot via imaging studies such as a sonogram or a CT scan. This level of medical testing and intervention should be conducted in a hospital. Therefore, outpatient clinics should monitor patients post-infusion and refer patients with complications to the nearest hospital.
Clotting or thromboembolism is a potential side effect from infusion of poorly characterized MSC therapeutics, especially when used at high doses with poor oversight. Under unfortunate circumstances, this can lead to disability and even death if no adequate countermeasures are readily available. Consequently, in order to guarantee the optimal safety of patients receiving MSC therapeutics, Guido Moll and colleagues recommend: “Improved MSC minimal criteria to maximize patient safety: a call to embrace tissue factor (TF/CD142) and hemocompatibility assessment of MSC products”.
References
- Moll G, Ankrum JA, Olson SD, & Nolta JA. Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products. Stem Cells Translational Medicine 2022; 11:2-13.
- Moll G, et al. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Frontiers Immunology 2020; 11:1091.
- Moll G, et al. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med 2019; 25(2):149-163.
- Moll G. et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012; 30(7):1565-1574.
- Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284(5411):143-147.
- Pittenger MF, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regenerative Medicine 2019; 4:22.
- Kabat M, Bobkov I, Kumar S, & Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Translational Medicine 2020; 9(1):17-27.
- Jovic D, et al. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Reviews and Reports 2022;
- CellTrials.org MSC Trials 2011-2020
- Turner L. The American stem cell sell in 2021: U.S. businesses selling unlicensed and unproven stem cell interventions. Cell Stem Cell 2021; 11:1891-1895.
- Caplan H, et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Frontiers Immunology 2019; 10:1645.
- Levy O, et al. Shattering barriers toward clinically meaningful MSC therapies. Science Advances 2020; 6:eaba6884.
- Ringdén O, Moll G, Gustafsson B, & Sadeghi B. Mesenchymal Stromal Cells for Enhancing Hematopoietic Engraftment and Treatment of Graft-versus-Host Disease, Hemorrhages and Acute Respiratory Distress Syndrome. Frontiers Immunology 2022; 13:839844
- World Health Organization. The top 10 causes of death. WHO website Updated 2019-12-09
- Lalu MM, et al. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2012; 7(10):e47559.
- Lalu MM, et al. Safety of cell therapy with mesenchymal stromal cells: An updated systematic review and meta-analysis of randomized controlled trials (SafeCell update). Cytotherapy 2018; 20(5):S53-S54.
- Thompson, M. et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. eClinicalMedicine 2020; 19:100249.
- Cyranoski D. Korean deaths spark inquiry. Nature News Report 2010; 468(7323):485.
- Jung, J.W. et al. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei medical journal 2013; 54(5):1293-1296.
- Wu, Z. et al. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant Proc 2017; 49(7):1656-1658.
- CDC. Venous Thromboembolism: Know the Risks, Signs & Symptoms of Blood Clots Web
- Bianco P, Robey PG, & Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2008; 2(4):313-319.
- Bianco P, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature Medicine 2013; 19:35-42
- Krampera M, & Le Blanc K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021; 28(10):1708-1725.
- Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008; 3(3):301-313.
- Silva Couto P. Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: lessons learnt and what the future holds. Biotechnology Advances 2020; 45:107636.
- Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8(4):315-317.
- Wallin RF. A Practical Guide to ISO 10993-4: Hemocompatibility. Medical Device and Diagnostic Industry MDDIonline Published 1998-11-01


