Jesteś tutaj
Grafix and Therapeutic Value of Placental Tissues: Please, Do Not Discard!

 The placenta is a multifunctional organ that is responsible for the development and protection of the fetus during pregnancy. At full term pregnancy, the placenta has maternal and fetal (baby’s) compartments. The fetal part of the placenta is comprised of the amniotic and chorionic membranes and umbilical cord (Fig. 1A).
The placenta is a multifunctional organ that is responsible for the development and protection of the fetus during pregnancy. At full term pregnancy, the placenta has maternal and fetal (baby’s) compartments. The fetal part of the placenta is comprised of the amniotic and chorionic membranes and umbilical cord (Fig. 1A).
Placental membranes have been used in medicine for over a century. The first scientific evidence on the use of fresh amniotic membrane for burns and wounds was published by Dr. Davis in 1910 in the Johns Hopkins Hospital Reports. Although his experience was rather negative he attributed it to a wrong technique and wrote: “The material is well worth a trial, and if a technic is developed by which favorable results can be obtained, it may be of great use1.” Later, his statement was confirmed by multiple studies reporting positive results with the amniotic membrane for management of burns and chronic wounds, eye diseases, oral and ear surgeries, and other applications2.
Therapeutic benefits of placental membranes are attributed to their composition (collagen-rich matrix with tissue nature growth factors and stem cells) and properties (anti-inflammatory, antibacterial, anti-scarring) that are supportive of tissue repair and regeneration (Fig. 1B). Another unique feature of placental membranes is the lack of components that will be recognized by a patient’s immune cells as “foreign” and therefore rejected after implantation. This allows a “universal” use of placental tissues, without matching between donors and patients.
Despite their benefits, placental membranes cannot be stored for a long time after collection (short shelf life), which limits their use. The goal of placenta preservation is to create an “off the shelf” product which protects the placental matrix and growth factors, and contains viable (living) stem cells. Studies show that alterations in the structure of placental tissue decrease the tissue functionality3.
Osiris Therapeutics has developed a proprietary cryopreservation method that retains the native structure of all placental components and can be stored at -80°C for years. Viable cryopreserved placental membranes (vCPMs) retain components of fresh placental membranes that are known to aid natural wound repair, including collagen rich matrix, endogenous growth factors and tissue viable cells3-6. There are two vCPM products commercially available from Osiris: Grafix Prime (amniotic membrane) and Grafix Core (chorionic membrane).
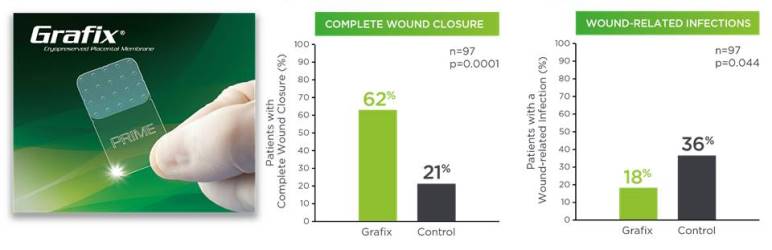
Figure 2 shows Grafix clinical results, in terms of higher wound closure rates and fewer wound related infections.12 Both Grafix products show positive results for a broad variety of clinical applications.
The treatment of chronic diabetic foot ulcers (DFUs) is a leading application for any wound dressing. There are currently 29.1 million Americans with type 2 diabetes7. DFUs are the most common complication of diabetes, with an estimated treatment cost of $15 billion per year, which puts an enormous burden on our society8. Diabetes adversely impacts wound healing, and often DFUs do not respond standard treatments developed by professional societies including American Diabetes Association and Wound Healing Society. Furthermore, non-healing DFUs have high risk of wound infections, hospitalizations and amputations9-10.
Clinical studies show that the vCPMs in Grafix are beneficial to treatment of chronic DFUs11-15. A prospective, multicenter, randomized, single-blinded clinical trial clinical trial compared Grafix to standard of care (SOC, the control group) for 97 patients with DFUs who were followed for 12 weeks. Lavery et al (2014) reported that Grafix demonstrated a significantly higher wound closure rate compared to control (62% vs 21.3%) and there were fewer wound-related infections (18% vs 36.2%) in the Grafix group. Based on these results, a cost effectiveness study estimated that Grafix saves approximately $14,000 per patient16.
 Another prospective multicenter clinical trial tested Grafix for complex DFUs characterized by exposed tendon, muscle, capsule or bone, in patients with multiple co-morbidities13. This study enrolled 31 patients who are typically excluded from clinical trials due to the severity of their wounds and the presence of co-morbidities that are known to be associated with poor wound healing. The mean wound area was 14.6 cm2, and mean duration was 7.5 months. All of the chronic wounds in this study had failed standard of care and 67.7% had failed one or more advanced wound care modalities prior to Grafix treatment. Grafix was applied weekly up to 16 weeks. Almost all patients (96.3%) developed granulated tissue in their wound bed (a precursor to wound closure) and 59.3% closed their wounds.
Another prospective multicenter clinical trial tested Grafix for complex DFUs characterized by exposed tendon, muscle, capsule or bone, in patients with multiple co-morbidities13. This study enrolled 31 patients who are typically excluded from clinical trials due to the severity of their wounds and the presence of co-morbidities that are known to be associated with poor wound healing. The mean wound area was 14.6 cm2, and mean duration was 7.5 months. All of the chronic wounds in this study had failed standard of care and 67.7% had failed one or more advanced wound care modalities prior to Grafix treatment. Grafix was applied weekly up to 16 weeks. Almost all patients (96.3%) developed granulated tissue in their wound bed (a precursor to wound closure) and 59.3% closed their wounds.
In summary, preparations of viable placental tissue such as Grafix have proven therapeutic value for the management of chronic wounds. Unfortunately, the majority of placentas are disposed as biological waste. Education, increased awareness, and establishment of placenta donation and collection programs at hospitals can help to provide more tissues for both research and clinical use.
References:
- Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910; 15:307.
- Stern W. The grafting of preserved amniotic membrane to burned and ulcerated skin surfaces substituting skin grafts. JAMA. 1913; 13:973–974.
- Johnson A, et al. Understanding the Impact of Preservation Methods on the Integrity and Functionality of Placental Allografts. Annals of Plastic Surgery. 2017.
- Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care (New Rochelle). 2015; 4:329–338.
- Duan-Arnold Y, Gyurdieva A, Johnson A, et al. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care (New Rochelle). 2015; 4:523–533.
- Duan-Arnold Y, Uveges TE, Gyurdieva A, et al. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care (New Rochelle). 2015; 4:513–522.
- Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention, 2011
- Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Medical, drug, and workloss costs of venous leg ulcers. Value Health. 2013; 16:A73.
- Markowitz JS, Gutterman EM, Magee G, Margolis DJ. Risk of amputation in patients with diabetic foot ulcers: a claims-based study. Wound Repair Regen. 2006; 14:11–7.
- Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999; 22:692–695.
- Regulski M, Jacobstein DA, Petranto RD, Migliori VJ, Nair G, Pfeiffer D. A retrospective analysis of a human cellular repair matrix for the treatment of chronic wounds. Ostomy Wound Manage. 2013; 59:38–43.
- Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, et al. The efficacy and safety of GrafixVR for the treatment of chronic diabetic foot ulcers: results of a multicentre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014; 11:554–60
- Frykberg RG, Gibbons GW, Walters JL, Wukich DK and Milstein FC. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane. Int Wound J 2016. 14:569–577.
- Suzuki K, Michael G, Tamire Y. Viable intact cryopreserved human placental membrane for a non-surgical approach to closure in complex wounds. Journal of Wound Care. 2016 25:Sup10, S25-S31
- Smedley J, Michael G, Tamire G. The International Journal of Lower Extremity Wounds. 2016; 15:4:360–365
- Nuccio EJ, et al. Innovative Treatment of Chronic Diabetic Foot Ulcer in a Controlled Randomized Clinical Trial Produces Fewer Adverse Events, Faster Wound Closure, and Lower Costs. J Clin Diabetes Pract. 2016;1:3

 Osiris Therapeutics, Inc., based in Columbia, Maryland, is a world leader in researching, developing, and marketing regenerative medicine products that improve the health and lives of patients and lower overall healthcare costs. Having developed the world's first approved stem cell drug, the Company continues to advance its research and development in biotechnology by focusing on innovation in regenerative medicine — including bioengineering, stem cell research and viable tissue based products. Osiris has achieved commercial success with products in wound care, orthopedics, and sports medicine, including Grafix®, Stravix™, BIO4™, and Cartiform®. More information can be found on the Company's website,
Osiris Therapeutics, Inc., based in Columbia, Maryland, is a world leader in researching, developing, and marketing regenerative medicine products that improve the health and lives of patients and lower overall healthcare costs. Having developed the world's first approved stem cell drug, the Company continues to advance its research and development in biotechnology by focusing on innovation in regenerative medicine — including bioengineering, stem cell research and viable tissue based products. Osiris has achieved commercial success with products in wound care, orthopedics, and sports medicine, including Grafix®, Stravix™, BIO4™, and Cartiform®. More information can be found on the Company's website,