You are here
2022 Update: How many clinical trials use cord blood or cord tissue?
Source | # Cumulative Trials | # Recruiting Trials |
Cord Blood | 276 | 110 |
Cord Tissue | 464 | n/a |
Other Perinatal | 151 | n/a |
From the beginning of advanced cell therapy with cord blood until the end of 2021, 276 cord blood clinical trials have been registered worldwide. We checked the status of these trials in June 2022 and found that 110 cord blood trials, both advanced therapy and traditional transplants, are recruiting at 281 locations. We have updated our portal of recruiting cord blood trials to enable patients and their families to look up these recruiting trials by the diagnosis treated.
We have also updated our statistics on advanced cell therapy with cord tissue, as well as other perinatal sources such as the placenta and amniotic membrane. Cumulatively through the end of 2021, there have been 464 trials with cord tissue alone and 151 trials with other perinatal tissues and combinations of perinatal tissues. Annual numbers of trials with cord tissue have grown more than four-fold over the time span from 2015 to 2020. In 2021 there were 30 trials registered for cord blood and 96 for cord tissue. We have not updated our portal of recruiting cord tissue trials because it has simply become too time consuming to check the status of so many trials.
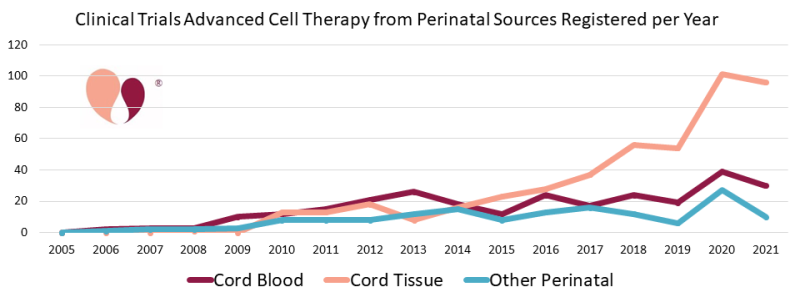
The Coronavirus pandemic has actually been a boon to perinatal cell therapy. We have compiled statistics showing that there was a roughly 25% bump in advanced cell therapy clinical trials during 2020, that most of this bump came from cell therapy efforts to treat COVID-19, and among those trials at least 40% relied on cells from perinatal sources. Meanwhile, the pandemic also caused many previously recruiting clinical trials to close. While updating the portal of recruiting cord blood trials, we found many cases of trials that simply did not have a status update, and we removed any trial from prior to 2019 that lacked an update.
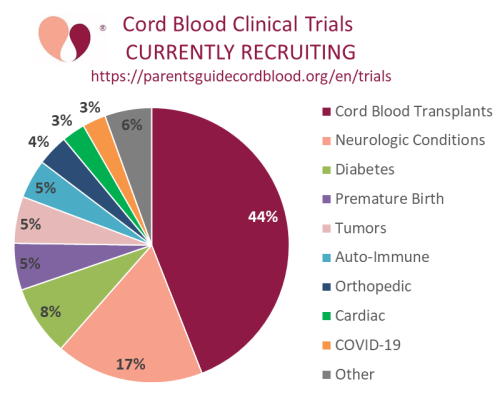 Our second graph is a pie chart of the diagnosis categories covered by the currently recruiting cord blood clinical trials. As in previous years, more than half the trials are for transplants of hematologic disorders (both malignant and metabolic disorders) or for neurologic conditions (this includes cerebral palsy and autism).
Our second graph is a pie chart of the diagnosis categories covered by the currently recruiting cord blood clinical trials. As in previous years, more than half the trials are for transplants of hematologic disorders (both malignant and metabolic disorders) or for neurologic conditions (this includes cerebral palsy and autism).
Over the years that we have been compiling clinical trials with cells from cord blood, the nature of these trials has evolved in some unexpected ways.
- We now have a handful of trials based on placental blood included in the category of cord blood. We felt this inclusion makes sense, since in utero it is the same blood supply that circulates between the baby, the umbilical cord, and the placenta.
- We originally started tracking trials of “manipulated” cord blood as a way to label trials with expanded cord blood. However, as a couple of these expanded products are moving closer to market approval, fewer trials of competing products are being registered. Meanwhile, clinical trials that use cord blood as a source to derive cells for immunotherapy have burst into importance. For example, during the year 2020, 40 trials of advanced cell therapy with cord blood were registered, and 14 (35%) of them were deriving Natural Killer (NK) cells from the blood.
- There continue to be about five trials per year that derive Mesenchymal Stromal Cells (MSC) from cord blood, and these trials are almost all in Asian countries.
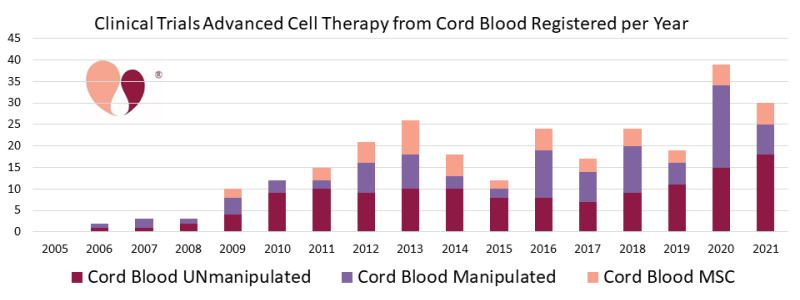
Looking towards the future, we expect that trials which use cord blood as a source of immunotherapy cells will be an ongoing feature of the 2020’s. We also want to call out that significant numbers of researchers are now exploiting cells from the placenta and amniotic membrane. In some clinical trials these birth tissues are used as another variation on culturing MSC, but in other cases they rely on epithelial cells in the amniotic membrane or use the entire membrane as a tissue engineering scaffold. Recently there have been a handful of trials each year that employ genetically modified MSC, and we anticipate this will be a growth field that will employ a portion of the perinatal MSC in years to come.